Spectrophotometers empower manufacturers across numerous industries to capture accurate color and spectral data. These measurements support regulatory compliance, conformance with industry standards, and quality assurance that keeps customer satisfaction and loyalty high. Modern solutions offer measurement through either transmission or reflectance optical configurations.
Learning more about these approaches, their ideal substrates and applications, and their differences will guide you to the best solution for your color measurement needs.
Understanding Color Measurement
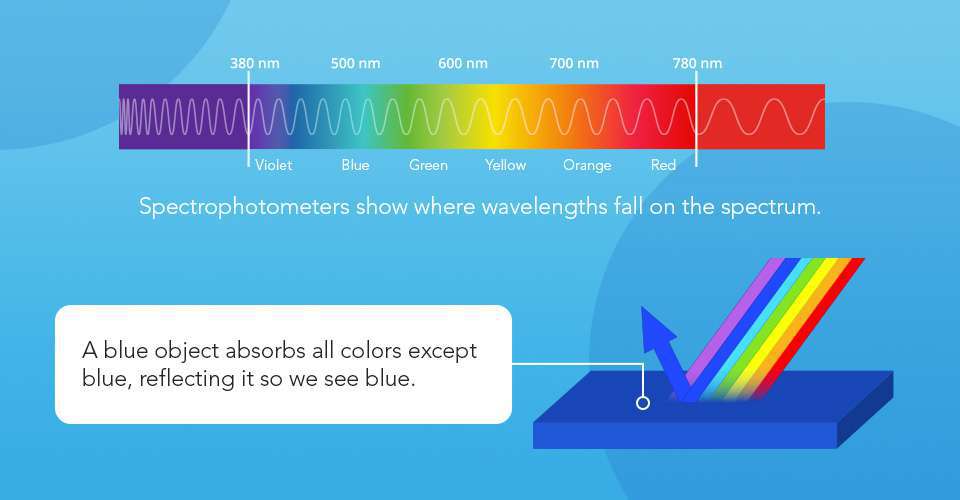
Light is the foundation of color measurement, with its wavelengths in the visible spectrum ranging from violet at 380 nanometers to red at 780 nanometers. The energy a light source provides varies across the spectrum depending on the light source. When it encounters matter, its wavelengths are either reflected, absorbed, refracted, transmitted, scattered, or diffracted. The object's chemical makeup helps determine what photons it will absorb versus those it emits or transmits. These emitted or transmitted wavelengths then enter the human eye, stimulating its photoreceptors. Emitted or transmitted wavelengths are the colors our eyes ‘see’ depending on where the energy falls within the visible spectrum.
Color perceptions vary between humans, making visual comparisons unreliable and underscoring the need for scientific color measurement solutions. Spectrophotometers simplify the task by analyzing and quantifying color in widely accepted color spaces like CIELAB and CIE XYZ. Transmission and reflectance are the two primary configurations spectrophotometers use to capture and report color and spectral data.
Color Measurement Through Transmission
When spectrophotometers measure color by transmission, they pass light directly through the sample. An optical sensor on the opposite side of the sample collects the energy for analysis and reporting.
The Science Behind Transmission Measurement
Measuring wavelengths through the transmission of color quantifies them into a ratio called transmittance. In scientific terms, transmittance is the percentage of incoming light that passes through a sample. The sample will also absorb a certain amount of the energy, requiring precise calculations using the Beer-Lambert Law, which describes how light is absorbed when it passes through matter.
Transmission measurements typically need at least 30% of the source light to pass through the sample with the light source at a perpendicular angle.
Key Considerations for Choosing Transmissive vs. Reflective Measurement
Transmission measurements work for transparent to translucent materials that allow 30% or more of the light to pass through — even if the surface is textured, like etched glass.
Opaque samples, which don’t let enough light through, are better measured using reflectance.
Applications for Transmission Color Measurement
Common applications for transmission color measurement include quantifying color in:
- Foods and beverages: Products ranging from edible oils to fruit juices and food dyes are compatible with transmittance measurement.
- Plastics: This approach is widely used in the plastics industry for food and beverage containers, transparent sheets and film, eyeglass lenses, and medical equipment like syringes, IV bags, and other clear medical devices.
- Chemicals: Use this method to quantify color in motor oils, lubricants, and petrochemicals.
- Pharmaceuticals: Injectable solutions and liquid medicines to monitor purity and detect any unwanted color changes.
Transmittance is also the most reliable color measurement approach for everyday products like clear liquid detergents and ophthalmic lense