Spectrometers or spectrophotometers are valuable tools companies use to ensure the quality and consistency of the products they make and sell. They can tell us if the oil is contaminated or if the fruit is ripe. They ensure that your favorite tomato ketchup is the same red, batch to batch. They provide the safety and efficacy of the medications you take. They are a valuable tool to ensure corporate brand integrity and that consistency and performance remain intact across an entire warehouse of products. To understand how spectrophotometers work, we need to comprehend the science of spectrophotometry.
Spectrophotometry is the science of how light interacts with matter. The objects we see daily are different forms of matter – solids, liquids, and gases. You may be surprised to learn that matter does not possess color. An apple appears red because of how light interacts with the chemical composition of the apple. When light strikes matter, some wavelengths are absorbed, and some are emitted. We see and perceive the emitted light as ‘color.’
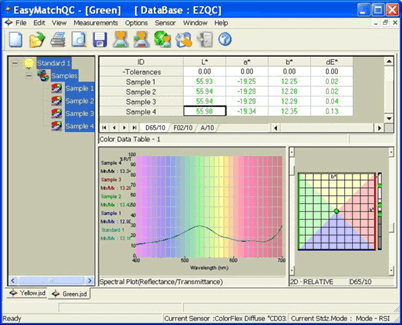
Spectrophotometers mimic this visual observing condition, providing information about the color of materials that is meaningful and actionable, allowing companies to ensure color accuracy and consistency across their range of products and brand colors.
So, how does all this happen from a spectrophotometer, and how do we use it? The science behind this tool is robust and can be utilized in several different ways. To clarify, we’ve gathered information on how spectrophotometers contribute to the color management of various products and technologies, so let’s dive in.
What Is a Spectrophotometer?

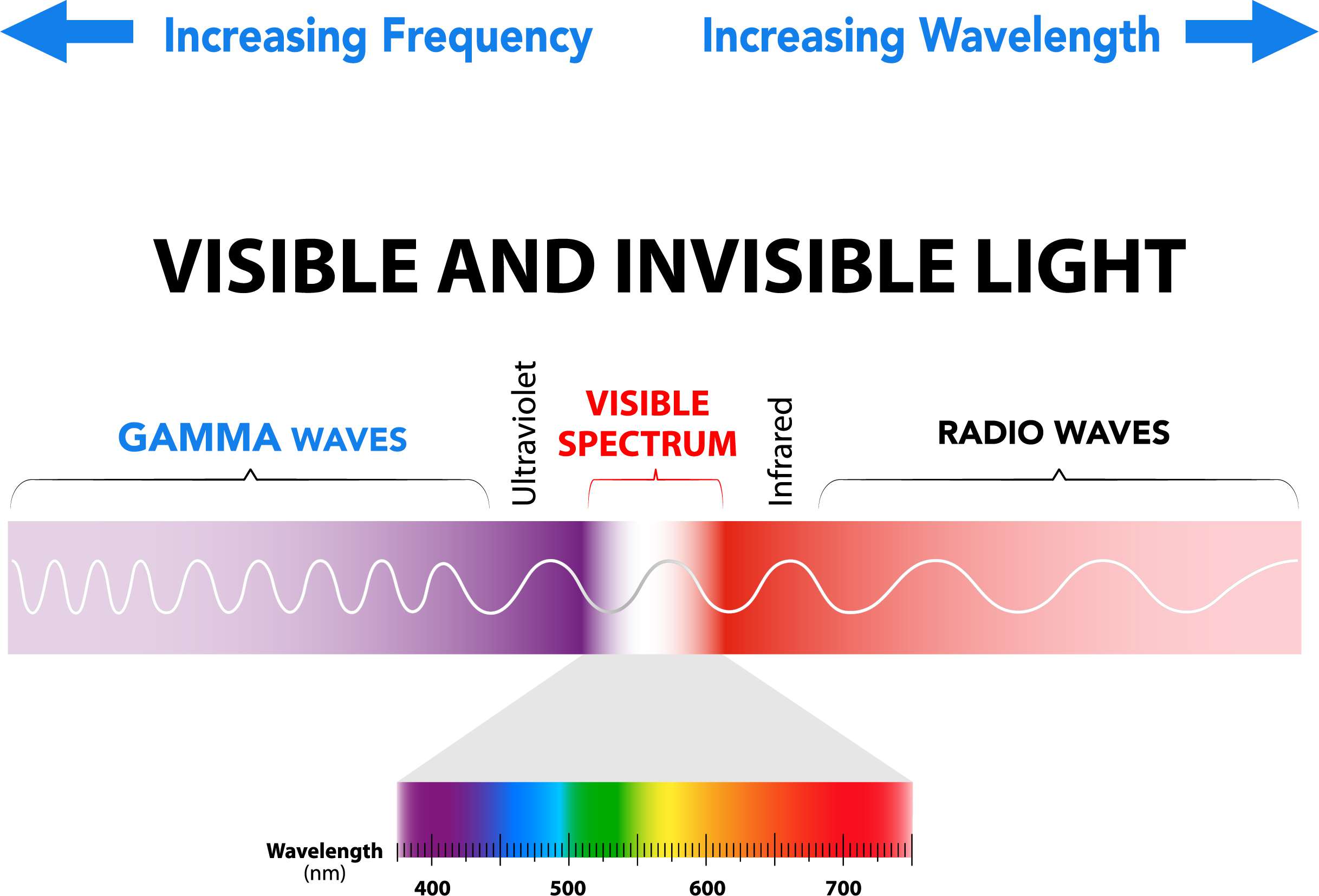
You may remember from chemistry class that light is a form of electromagnetic radiation, like microwaves and gamma rays. When we talk about the spectrum of light, we’re talking about a spectrum of energy, where different energy levels create what we perceive as other colors. The colors of the rainbow follow the progression of visible energy, with red being the lowest and violet being the highest. Materials that absorb all visible light appear black, while those that do the opposite appear white. In between those two are materials that emit certain light energies and absorb others, displaying specific colors.
A spectrophotometer is essentially a calibrated light counter.
“Spectro” refers to the fact that light is dispersed into individual wavelengths in the electromagnetic energy spectrum. Some of that energy is in the ultraviolet and visible spectrum, which spectrophotometers can read, while other spectrometers can measure infrared radiation.
“Photometer” measures light intensity at specific wavelengths and is scaled from 0 to 100. Zero equals total darkness, and 100 is perfect white. Some properties, like fluorescence, make it possible for this scale to go over 100, so most spectrophotometers can reach 150 or 200.
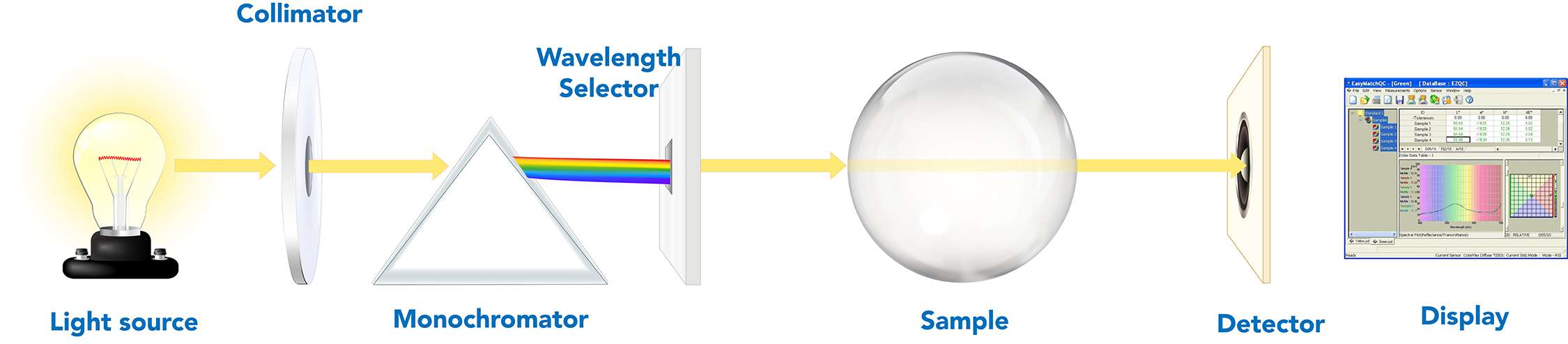
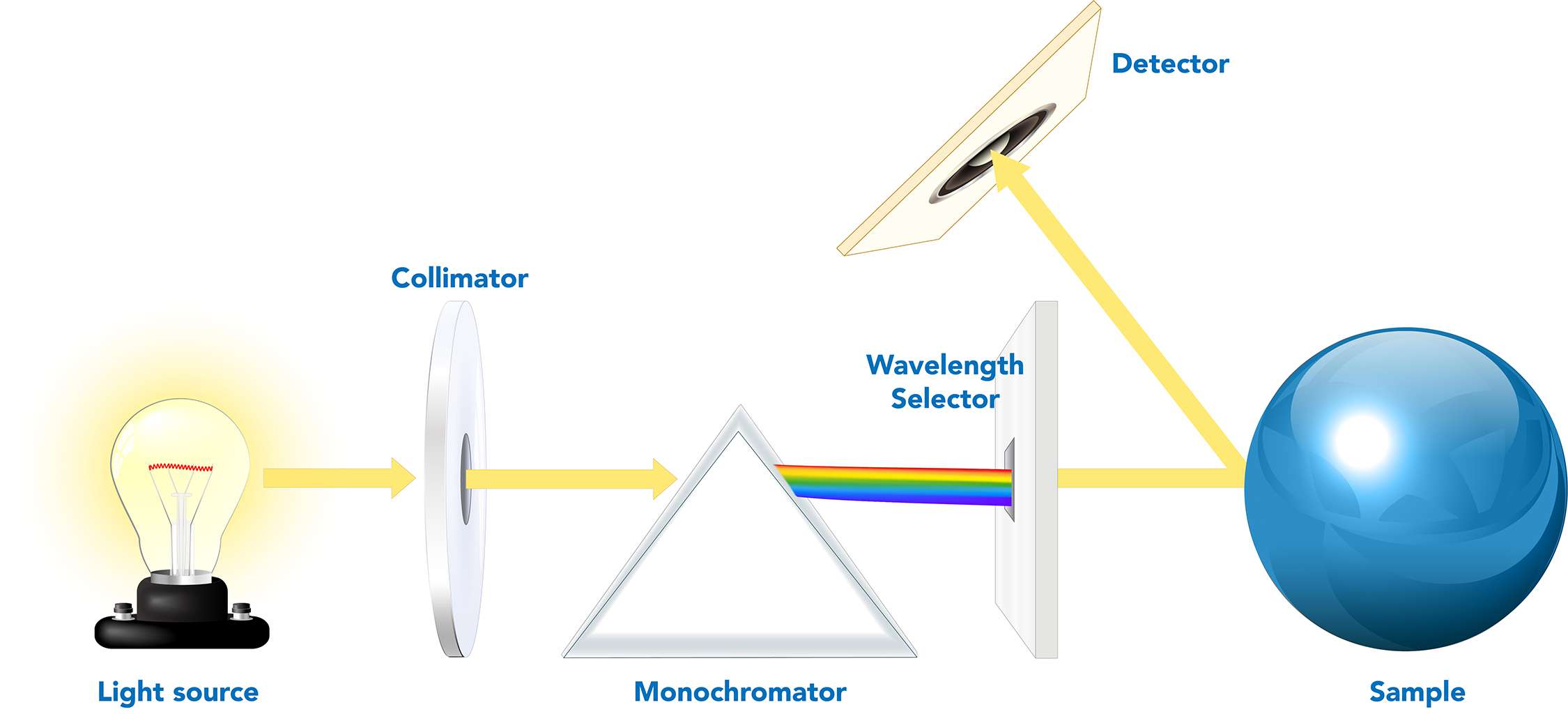
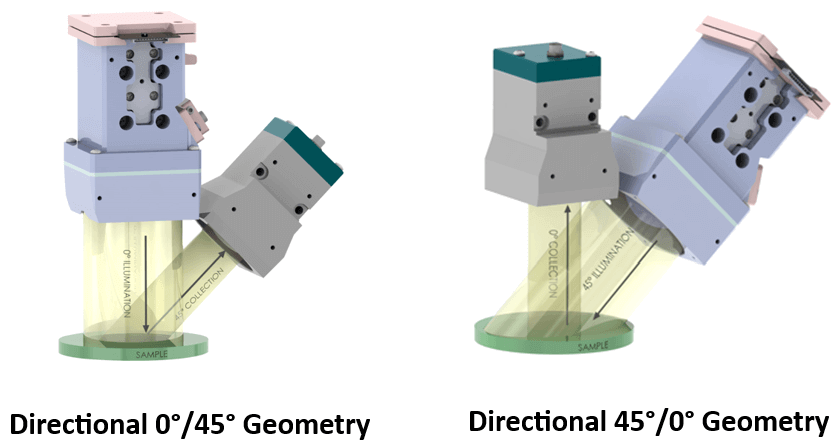
Spectrophotometers are used to measure the color of materials. Materials can be solid, liquid, opaque, translucent, or transparent. Different methods are used to measure these materials, depending on their form and transparency. Opaque materials are measured using reflectance spectrophotometers, which measure the amount of light reflected from a sample. In contrast, transparent materials use transmission spectrophotometers, which measure the amount of light that passes through the material. Regardless of the method used, all spectrophotometers share the same basic technology and optical design:
- A controlled light source to illuminate the material.
- A lens to collimate the light to the monochromator.
- A monochromator that separates the light into its constituent color wavelengths.
- A wavelength selector.
- A detector that quantifies the light emitted from the sample.
- A display that provides results.